페이지 정보
3D Balance Trainer「3DBT-11」
Product Features
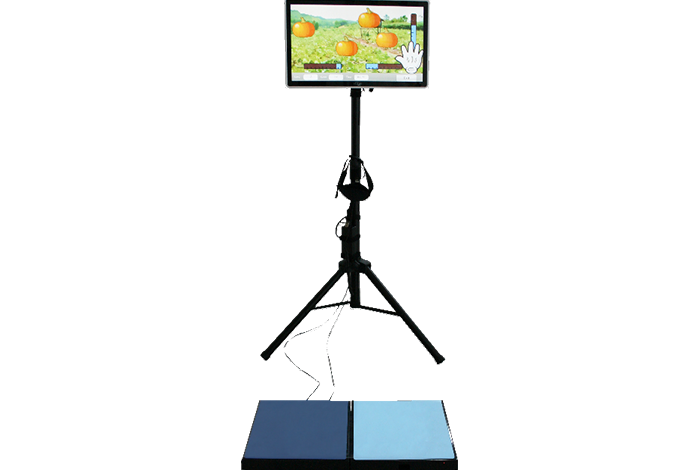
Balance trainer by horizontal shift of center of gravity
Balance trainer by vertical shift of center of gravity (world's first)
Evaluation and training possible with one device
An accurate and right rehabilitation based on the medical science
Training equipment for an excellent rehabilitation effect in a short time of use
Same function as 3DBT-12 but smaller size &economic model
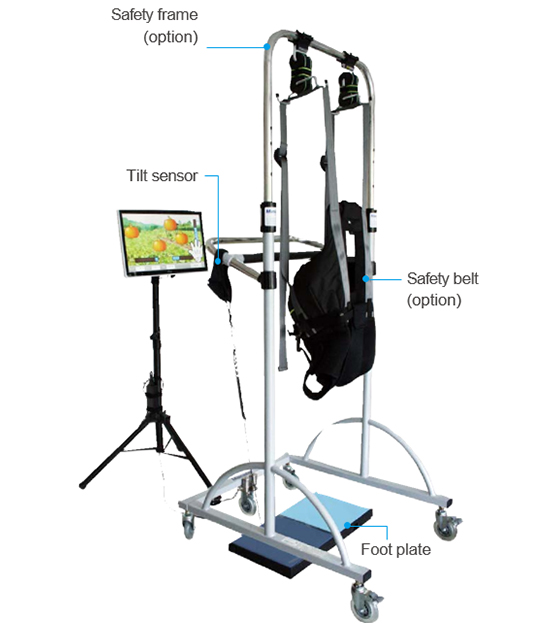
Safety frame itself can be used for gait training
Remote diagnosis, control, update and monitoring for maintenance
본문
Product Specifications
Footplate Size | 620(W) X 310(L)mm |
Bag Size | 670(W) X 520(L) X 270(H)mm |
Option Size | 730(W) X 850(L) X 1,950(H)mm |
Installation place(option) | 730(W) X 2,000(L) X 1,950(H)mm |
Product weight | 18Kg |
Bag weight(including product and packaging) | 23kg |
Max. bearable weight | 150Kg |
Angle of knee joints | -70° ~ +70° |
Power/Consumption | AC 220V, 50/60Hz / 36W |
Monitor | 21.5˝ Full HD touch screen |
Composition | Footplate, All-in-one PC, Monitor stand, Tilt sensor 2EA, Assessment & Training Contents Option : Safety frame, Safety belt |
Additional Description
This equipment is a medical device to train horizontal weight shift and vertical flexion of knee for the patient who needs rehabilitation of lower extremities.
It can assess the balance of a patient by measuring the weight shift of left/right by the sensor in the foothold and the motion range of flexion of knee joints by the tilt sensor attached on the leg.
Patient can train himself actively playing the game pleasantly and check the improvement with the accumulated data. 3DBT-11 has the same basic function and effect as 3DBT-12, however, with simplified design and reduction of size for the cost reduction.
We recommend to use this device for personal use at home, at rehabilitation clinic that needs space saving, public health center and nursing home.
Medical Equipment Certificates
KFDA Product name : System, isokinetic testing and evaluation
KFDA Manufacture Registered No. 3399
KGMP Certificated / ISO13485 Certificated
FDA Device name : SYSTEM, ISOKINETIC TESTING AND EVALUATION
FDA Registration No. 3011806255
FDA Listing No. D245554
Korea Patents (No. 10-1262875, No. 10-1262876)